
Flouride is a beneficial ion known for being added to toothpastes and water to improve enamel strength. When taken in excess however it can actually mineralize and cause impaired organ function. Flouride mineralization can occur in various tissues such as the kidneys, thyroid, brain, and causes deformities in bones found in the legs and spine. As such when living in a country that experiences flouride leaks in drinking water it is important to detect excess quantities.
Various methods of detection exist which make use of chemical detection molecules which provide visual and portable solutions. These detection molecules however have varying degrees of accessibility and effectiveness. One method involves a dissolving chemical which emits light once dissolved by flouride containing water. Another involves an active light emitting substance which becomes inactive with a specific ratio of flouride ions in solution. While both are useful, they can only be used once when the detection molecule dissolves or binds permanently when used. Ebrahim and colleagues note that while covalent bonding can be specific they prevent detection molecules from being reused. On the other hand electrostatic bonding is not used due to its lack of specificity but they allow the detection molecule to be reused.
Ebrahim et. Al. therefore took this into account when constructing a specific detection molecule which could be reused, stable in solution, and safe. This lead them to construct SION-105, a metal-organic framework molecule (MOF), which makes use of a specific non-permanent detection molecule. The name MOF refers to a carbon-based molecule containing a metal and these can be useful in creating stable active molecules. SION-105 takes advantage of europium, a lanthanide transition metal, to emit a reddish hue when the boron atom reacts to light energy.
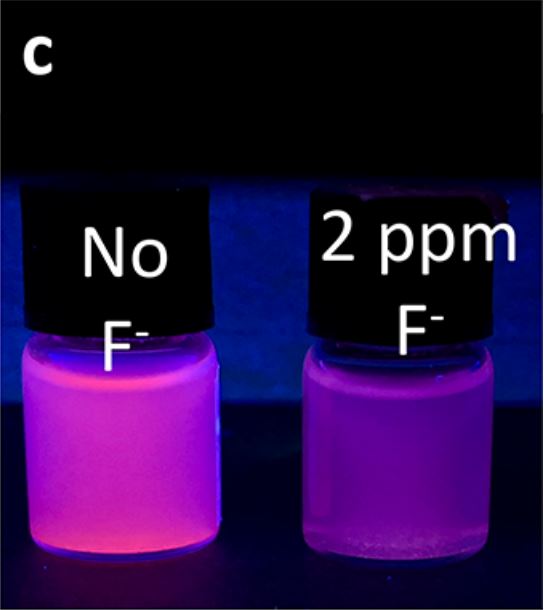
SION-105 demonstration Figure 2C by Ebrahim et. Al.
When it comes in contact with flouride, boron is prevented from absorbing the energy needed for europium to emit light. The boron specifically reacts with flouride within the carbon framework where only flouride can reach it. Not only does the carbon framework allow the detection molecule to be specific, but also prevents any permanent or covalent bonding between the molecules. This means that the transient electrostatic bond can be undone in this case simply by ethanol.
Due to the various characteristics of the SION-105 molecule, it can be a reusable and reliable way to detect excess amounts of flouride. According to Ebrahim et. Al. these important qualities arise due to rigidity of the carbon structure limiting boron-flouride bonding, steric hinderance creating specificity, and electrostatic reversibility with ethanol. They also mention that SION-105 is relatively easy to produce and with high yields. The molecule is also polymerized or chained in repeated segments to improve its effects. It can also be further improved by the solution it is in as only a small amount of it is needed in this repeat version to visually detect flouride.
This detection molecule stands to replace alternatives and help those who are endangered by excess flouride in drinking water. Its accessibility can provide a great solution and with enough knowledge about drinking water, steps can be made to improve the resources available to small towns for example. Due to the fact that drinking water is so important it is crucial to have an accessible and affordable method of detection for harmful substances.
In Depth
In general the structure and content of chemistry articles has surprised this reader. They seem to be shorter and to the point which lines up with the goals of this blog itself. It will be acknowledged that the material contained in chemistry research might be in and of itself, conducive to the short and direct writing style of their research article reports. The study itself however is extremely invaluable to anyone who wishes to continue this line of work as they include their methods and reasoning. Ebrahim et. Al. provide detailed instructions on optimizing a solution of SION-105 and explain the need for it. While they do provide solid arguments for the benefit of their solution over others, it would also be extremely useful and transparent for them to provide drawbacks or disadvantages. In addition, it is still necessary to show explicit tests for its safety when consumed in clinical trials and such. It does however seem possible to quantify the amount of flouride in solution given the SION-105 system given that in Figure 2 of their paper the bonding occurs along a curve while also being a visual gradient of change. It may be possible to calculate the concentration of flouride ions using a method similar to titrations and the like. Overall a very helpful discovery and a solid presentation of its benefits. More papers could benefit from being transparent and to the point as it is too much to expect all papers to be minimal in scientific jargon and direct.
Chinh Le Duc. https://unsplash.com/@mero_dnt
Ecole Polytechnique Fédérale de Lausanne. “New device simplifies measurement of fluoride contamination in water.” ScienceDaily. ScienceDaily, 11 February 2019. http://www.sciencedaily.com/releases/2019/02/190211083227.htm
Fatmah Mish Ebrahim, Tu N. Nguyen, Serhii Shyshkanov, Andrzej Gładysiak, Patrick Favre, Anna Zacharia, Grigorios Itskos, Paul J. Dyson, and Kyriakos C. Stylianou. Selective, Fast-Response, and Regenerable Metal–Organic Framework for Sampling Excess Fluoride Levels in Drinking Water. Journal of the American Chemical Society 2019 141 (7), 3052-3058 DOI: 10.1021/jacs.8b11907
Researcher App. https://www.researcher-app.com/
Unsplash. Researcher App. https://www.researcher-app.com/

You must be logged in to post a comment.